- Home
- 2. About this Manual
- 2.1 Purpose of the Manual
- 2.2 Navigating the Manual
- 2.3 Determining Changes to the Manual
- 2.4 Governance for Editing the Manual
- 2.5 Periodic Review and Maintenance
- 3. Quality and Customer Engagement
- 3.1 Quality
- 3.2 Customer Service Charter (Timeliness Guidelines)
- 3.3 Efficient Examination
- 3.3.1 Use of FERs (Earlier Search and Examination Reports)
- 3.3.2 General Approach to Examination
- 3.3.3 Reserving Opinion and Restricting the Search
- 3.3.4 Communicating with the Applicant and Third Parties
- 3.4 Assisting Unrepresented Applicants
- 3.5 Staff Delegations, and Restrictions on Providing Customer Assistance
- 4. Classification and Searching
- 4.1 Search Theory
- 4.2 Patent Classifications
- 4.2.1 Patent Classification Systems
- 4.2.1.1 International Patent Classification (IPC)
- 4.2.1.1.1 Structure of the IPC
- 4.2.1.1.2 Headings and Titles
- 4.2.1.1.3 Definitions, Warnings and Notes
- 4.2.1.1.4 Function-Oriented and Application-Oriented Places
- 4.2.1.1.5 References
- 4.2.1.1.6 Indexing Codes
- 4.2.1.2 Cooperative Patent Classification (CPC)
- 4.2.2 Principles of Classification
- 4.2.2.1 Invention Information and Additional Information
- 4.2.2.1.1 Application of Indexing Codes/2000 Series
- 4.2.2.1.2 Classifying in Residual Places
- 4.2.2.1.3 Places that cannot be the First Symbol
- 4.2.2.2 Classification Priority Rules
- 4.2.2.2.1 Common Rule
- 4.2.2.2.2 First Place Priority Rule
- 4.2.2.2.3 Last Place Priority Rule
- 4.2.2.2.4 Special Rules
- 4.2.2.2.5 Classifying a Combination of Technical Subjects
- 4.2.2.3 Classifying in Function-Oriented and Application-Oriented Places
- 4.2.2.4 Classifying Chemical Compounds
- 4.2.2.5 CPC Classification Rules
- 4.2.2.6 Classification using C-sets
- 4.2.3 Other Classification Information
- 4.2.3.1 Sub-Codes - Discontinued
- 4.2.3.2 The Australian Classification System - Discontinued
- 4.2.3.3 Indexing According to IPC Edition (2006) - Discontinued
- 4.2.3.4 Master Classification Database (MDC)
- 4.2.3.5 Recording Classification Symbols on Machine-Readable Records
- 4.2.3.6 Presentation of Classification Symbols and Indexing Codes on Patent Documents
- 4.3 Initial Search Considerations
- 4.3.1 Construction and the Inventive Concept
- 4.3.2 Earlier Search Results
- 4.3.3 Additional Searching
- 4.3.4 Top-Up Searching
- 4.3.5 Preliminary Search
- 4.3.6 Applicant and/or Inventor Name Searching
- 4.4 Development of the Search Strategy
- 4.4.1 Three Person Team (3PT)
- 4.4.2 Search Strategy Considerations
- 4.4.2.1 Independent Claims
- 4.4.2.2 Dependent Claims
- 4.4.2.3 Broad Claims
- 4.4.2.4 Reserving the Search
- 4.4.2.5 Controlled Language
- 4.4.3 Search Area
- 4.5 Conducting the Search
- 4.6 Recording the Search Details
- 4.7 Annexures
- Annex D - Search Information Statement
- Annex E - Examples and Instructions for completing the SIS for Sequence and Chemical Structure Searches
- Annex F - When to Complete the Search Information Statement (SIS)
- Annex N - Guidelines for Searching Indian TKDL
- Annex P - The Role of the Three Person Team (3PT) in Searching
- 4.8 User Guides
- 5. National
- 5.1 Procedures
- 5.2 Understanding Legislation
- 5.2.1 Modern Australian Law
- 5.2.2 Working with case law
- 5.2.3 Working with statute
- 5.2.4 Practical guide to interpreting legislation
- 5.3 Formalities and Forms
- 5.3.1 Formalities Checking
- 5.3.1.1 Formalities Required and Assessed at Filing
- 5.3.1.2 Credible Address for Service
- 5.3.1.3 Formalities Required and Assessed During Examination
- 5.3.2 Formal requirements of the Specification
- 5.3.2.1 Title of the Application
- 5.3.2.2 Abstracts
- 5.3.2.3 Requirements for Text, Pagination, Formulas, Equations, Drawings, Graphics, and Photographs
- 5.3.2.4 Substitute Pages to comply with formalities
- 5.3.2.5 Requirements for Amino Acid and Nucleotide Sequences
- 5.3.2.6 Scandalous Matter
- 5.3.3 Approved Forms (including patent request)
- 5.3.4 Signature Requirements for Forms and Other Documents
- 5.3.5 Return or Deletion of Documents
- 5.4 Entitlement
- 5.4.1 Who can file and who can be granted a patent
- 5.4.2 Statement of entitlement
- 5.4.3 Artificial Intelligence - Inventorship and Entitlement
- 5.4.4 Annex A - Examples of Legal Persons
- 5.4.5 Annex B - Examples of Organisations of Uncertain Status as Legal Persons
- 5.5 Construction of Specifications, Claims, and Claim Types
- 5.5.1 Purpose of Construction
- 5.5.2 Considerations Relevant to Construction of the Specification
- 5.5.2.1 Initial Considerations
- 5.5.2.2 The Addressee
- 5.5.2.3 The Role of Common General Knowledge
- 5.5.2.4 The Invention Described
- 5.5.3 Rules of Construction for a Specification
- 5.5.3.1 Words are Given Plain Meaning
- 5.5.3.2 Specification Read as a Whole
- 5.5.3.3 Purposive Construction
- 5.5.3.4 Dictionary Principle
- 5.5.3.5 Reject the Absurd
- 5.5.3.6 Description Construed as a Technical Document
- 5.5.3.7 Errors, Mistakes, Omissions
- 5.5.4 Claim Construction and Claim Types
- 5.5.4.1 Claims are Construed as a Legal Document
- 5.5.4.2 Presumption Against Redundancy
- 5.5.4.3 Omnibus Claims
- 5.5.4.4 Swiss Claims
- 5.5.4.5 Product by Process Claims
- 5.5.4.6 Parametric Claims
- 5.5.4.7 ‘For Use’, ‘When Used’ and Similar Wording in Claims
- 5.5.4.8 ‘Comprises‘, ‘Includes‘, ‘Consists of‘ and ‘Contains‘ and Similar Wording in Claims
- 5.5.4.9 Reference Numerals in Claims
- 5.5.4.10 Relative Terms
- 5.5.4.11 ‘Substantially‘, ‘About‘, ‘Generally’
- 5.5.4.12 Appended Claims
- 5.6 Examination
- 5.6.1 Relevant Dates, Definitions, Legal Standards and Other Prescribed Matters (e.g Publication)
- 5.6.1.1 Priority dates and Filing Dates
- 5.6.1.2 Effect of Publication
- 5.6.1.3 Definitions (Invention, Alleged Invention, Meaning of a Document etc.)
- 5.6.1.4 Balance of Probabilities Standard
- 5.6.1.5 Application of the Balance of Probabilities in Examination
- 5.6.2 Factors to consider before commencing examination
- 5.6.2.1 Request for Examination
- 5.6.2.2 Application in a State of Lapse?
- 5.6.2.3 Extension of Time Requested (s223 actions)
- 5.6.2.4 Payment of Fees
- 5.6.2.5 Translations of Specifications, Article 19 and Article 34 Amendments (Requirements for Certification, Poor Translations)
- 5.6.2.6 Obtaining Priority Documents
- 5.6.2.7 Excess Claims Fees and Invitation to Pay
- 5.6.2.8 Report Dispatch, Correction of Report etc.
- 5.6.2.9 Further Report Considerations
- 5.6.2.10 Convention applications
- 5.6.3 The Specification and Claims to Examine
- 5.6.3.1 Consideration of Amendments Made prior to examination
- 5.6.3.2 Claims are directed to a Single Invention (Unity)
- 5.6.3.3 Omnibus claims – References to the Descriptions or Drawings
- 5.6.3.4 Provisional specifications - Examination
- 5.6.4 Citations: Prior Art Base and Construction of Prior Art
- 5.6.4.1 Prior Art - What is Included (Definition From the Act, Publicly Available, Exclusions, Grace Period)
- 5.6.4.2 Construing a Citation
- 5.6.4.3 Level of Disclosure Required (Enabling Disclosure, Clear and Unmistakable Directions etc)
- 5.6.4.4 Single Source of Information, Combination of Documents
- 5.6.4.5 Third Party Notifications
- 5.6.4.6 Identifying and Raising Citations
- 5.6.5 Novelty, Whole of Contents, Grace Periods, Secret Use
- 5.6.5.1 Determining Novelty
- 5.6.5.2 Whole of Contents
- 5.6.5.3 Prior Use, Secret Use and Confidential Information
- 5.6.5.4 Novelty - Specific Examples
- 5.6.5.5 Selections
- 5.6.5.6 Issues Specific to Chemical Compositions
- 5.6.6 Inventive Step
- 5.6.6.1 Inventive Step Requirements
- 5.6.6.2 Information for Assessing Inventive Step
- 5.6.6.3 Tests for Inventive Step
- 5.6.6.4 Assessing Inventive Step
- 5.6.6.5 Indicators of Inventive Step
- 5.6.6.6 Issues Specific to Chemical Compositions
- 5.6.7 Full Disclosure, Sufficiency, Clarity and Support (S40 considerations)
- 5.6.7.1 Claims are Clear and Succinct
- 5.6.7.2 Clear and Complete Disclosure s40(2)(a)
- 5.6.7.3 Support for the Claims s40(3)
- 5.6.7.4 Difference Between ‘Clear and Complete Disclosure’ and ‘Support’
- 5.6.7.5 Best Method
- 5.6.7.6 Complete Disclosure Micro-Organisms and Other Life Forms (Budapest Treaty, Deposit Requirements)
- 5.6.7.7 Claims define the Invention
- 5.6.7.8 Annex A - Examples: Subsections 40(2)(a) and 40(3)
- 5.6.8 Patent Eligible Subject Matter (Manner of Manufacture, Usefulness)
- 5.6.8.1 General Principles-Assessing Manner of Manufacture
- 5.6.8.2 Alleged Invention
- 5.6.8.3 Fine Arts
- 5.6.8.4 Discoveries, Ideas, Scientific Theories, Schemes and Plans
- 5.6.8.5 Printed Matter
- 5.6.8.6 Computer Implemented Inventions, Schemes and Business Methods
- 5.6.8.7 Games and Gaming Machines
- 5.6.8.8 Mathematical Algorithms
- 5.6.8.9 Methods of Testing, Observation and Measurement
- 5.6.8.10 Mere Working Directions
- 5.6.8.11 Nucleic Acids and Genetic Information
- 5.6.8.12 Micro-Organisms and Other Life Forms
- 5.6.8.13 Treatment of Human Beings
- 5.6.8.14 Human Beings and Biological Processes for Their Generation
- 5.6.8.15 Agriculture and Horticulture
- 5.6.8.16 Combinations, Collocations, Kits, Packages and Mere Admixtures
- 5.6.8.17 New Uses
- 5.6.8.18 Other Issues e.g. Contrary to Law, Mere Admixtures
- 5.6.8.19 Useful (Utility)
- 5.6.8.20 Annex A - History of Manner of Manufacture
- 5.6.9 Acceptance, Grant and Refusal of Applications
- 5.6.9.1 Conditions and Time for Acceptance
- 5.6.9.2 Postponement of Acceptance
- 5.6.9.3 Extension of Acceptance Period
- 5.6.9.4 Revocation of Acceptance
- 5.6.9.5 Refusal of Acceptance-Specific Circumstances
- 5.6.9.6 Refusal of an Application
- 5.6.9.7 Continued Examination-Result of a Decision
- 5.6.9.8 Lapsing of an Application
- 5.6.9.9 Withdrawal of an Application
- 5.6.9.10 Double Patenting - S64(2) and 101B(2)(h) - Multiple Applications
- 5.6.9.11 Parallel applications (applications for both innovation and standard)
- 5.6.9.12 Register of Patents
- 5.6.9.13 Annex A - Example Bar-to-Grant Letter (Accepted Despite Multiple Inventions)
- 5.6.9.14 Acceptance and QRS Issues
- 5.6.10 Divisional Applications
- 5.6.10.1 Requirements to Claim Divisional Status
- 5.6.10.2 Priority Entitlement
- 5.6.10.3 Time Limits for Filing
- 5.6.10.4 Status of the Parent
- 5.6.10.5 Examination of Divisional Applications
- 5.6.10.6 Innovation Divisional Applications
- 5.6.10.7 Annex A - Procedural Outline to Divisional Examination
- 5.6.11 Patents of Addition
- 5.6.11.1 Conditions for Filing
- 5.6.11.2 Improvement or Modification
- 5.6.11.3 Differentiation from the Parent
- 5.6.11.4 Examination of Additional Applications
- 5.6.11.5 Amendment of the Parent
- 5.6.11.6 Annex A - Procedural Outline to Patents of Addition Examination
- 5.6.12 Preliminary search and Opinion (PSO)
- 5.6.12.1 Requests for PSO
- 5.6.12.2 PSO - Search and Examination Procedure
- 5.6.12.3 PSO - Report Requirements
- 5.6.12.4 Response to the PSO
- 5.6.13 Re-Examination
- 5.6.13.1 Commencing Re-Examination
- 5.6.13.2 Re-Examination Process
- 5.6.13.3 Completion of Re-Examination
- 5.6.13.4 Refusal to Grant or Revocation Following Re-Examination
- 5.6.14 Prohibition Orders- Applications Concerning Defence of the Commonwealth and/or involving Associated Technology (e.g. enrichment of nuclear material)
- 5.6.14.1 Effect of Prohibition orders
- 5.6.14.2 Applications Concerning Defence of the Commonwealth
- 5.6.14.3 Applications Concerning ‘Associated Technology’ (Chapter 15 Applications)
- 5.6.15 Innovation Patents
- 5.6.15.1 The Innovation Patent System
- 5.6.15.2 Types of Innovation Patents
- 5.6.15.3 Formalities Check for Innovation Patents
- 5.6.15.4 Examination of Innovation Patents
- 5.6.15.5 Determining Innovative step
- 5.6.15.6 Certification, Opposition, Ceasing/Expiring of Innovation Patents
- 5.6.15.7 Annex - Innovation Patent Certification Form
- 5.6.16 Annex A - Procedural Outline for Full Examination of a Standard Patent Application
- 5.6.17 Annex B - Examination of National Phase Applications: Indicators of Special or Different Considerations
- 5.6.18 Annex C - Applicant and Inventor Details as Shown on PCT Pamphlet Front Page
- 5.6.19 Annex D - Example of PCT Pamphlet Front Page
- 5.7 Amendments
- 5.7.1 What can be Amended and When
- 5.7.1.1 What Documents can be Amended?
- 5.7.1.2 Who can Request Amendments (incl consent of licensees/mortgagees)?
- 5.7.1.3 When can Amendments be Requested?
- 5.7.1.4 Requirements to provide Reasons for Amendments
- 5.7.1.5 Withdrawal of Amendments
- 5.7.1.6 Circumstances where an amendment cannot be processed (i.e. pending court proceedings, Application has been Refused)
- 5.7.1.7 Granting Leave to Amend
- 5.7.1.8 Fees Associated with Amendments
- 5.7.1.9 Annex A - Guidelines for Completing the Voluntary Section 104 Allowance Form
- 5.7.2 Amendment of the Patent Request and Other Filed Documents
- 5.7.2.1 Form of the Request to Amend the Patent Request
- 5.7.2.2 Non-Allowable Amendments to Patent Request
- 5.7.2.3 Changing the Applicant or Nominated Person
- 5.7.2.4 Converting the Application
- 5.7.2.5 Amendments to the Notice of Entitlement and Other Documents
- 5.7.3 Amendments-Provisional Applications
- 5.7.4 Amendments to Complete Specifications
- 5.7.4.1 Form of proposed amendments (statement of proposed amendments)
- 5.7.4.2 Allowability of Amendments Prior to Acceptance
- 5.7.4.3 Allowability of Amendments After Acceptance
- 5.7.4.4 Allowability of Amendments After Grant
- 5.7.4.5 Amendments not Otherwise Allowable
- 5.7.4.6 Opposition to Amendments
- 5.7.4.7 Annex A - Amended Claims Format
- 5.7.5 Amendments to Correct a Clerical Error or Obvious Mistake
- 5.7.5.1 Definition of Clerical Error
- 5.7.5.2 Definition of Obvious Mistake
- 5.7.5.3 Evidence required to prove a Clerical Error or Obvious Mistake
- 5.7.6 Amendments Relating to Micro-Organisms and Sequence Listings
- 5.7.6.1 Insertion or Alteration of Sec 6(c) Information
- 5.7.6.2 Amendments or Corrections of Sequence Listings
- 5.7.7 Amendments during Opposition Proceedings
- 5.7.7.1 Initial Processing of the Request to Amend During Oppositions
- 5.7.7.2 Considering the Amendments and Comments from the Opponent
- 5.7.7.3 Considering Amendments as a Result of a Hearing Decision
- 5.7.7.4 Amendments where Decision of the Commissioner is Appealed
- 5.7.7.5 Annex A - Section 104 Amendments During Opposition Proceedings: Check Sheet
- 6. International
- 6.1 International Searching
- 6.1.1 Procedural Outline - PCT International Search
- 6.1.2 Introduction- International Searching
- 6.1.2.1 Overview- International Searching
- 6.1.2.2 Overview-International Search Opinion (ISO)
- 6.1.2.3 General Procedures
- 6.1.2.4 Extent of Search
- 6.1.2.5 Minimum Documentation
- 6.1.2.6 Examination Section Procedures
- 6.1.2.7 Searching Examiner
- 6.1.2.8 Other Considerations
- 6.1.2.9 Copending Applications
- 6.1.3 Search Allocation and Preliminary Classification
- 6.1.4 Unity of Invention
- 6.1.4.1 Unity of Invention Background
- 6.1.4.2 Determining Lack of Unity
- 6.1.4.3 Combinations of Different Categories of Claims
- 6.1.4.4 Markush Practice
- 6.1.4.5 Intermediate and Final Products in Chemical Applications
- 6.1.4.6 Biotechnological Inventions
- 6.1.4.7 Single General Inventive Concept
- 6.1.4.8 A Priori and A Posteriori Lack of Unity
- 6.1.4.9 Issuing the Invitation to Pay Additional Search Fees
- 6.1.4.10 Unsupported, Unclear, Long and/or Complex Claim Sets with Clear Lack of Unity
- 6.1.4.11 Payment of Additional Search Fees Under Protest
- 6.1.4.12 Completing the Search Report
- 6.1.4.13 Time for Completing the Search Report
- 6.1.4.14 Reported Decisions
- 6.1.4.15 Other Decisions from the EPO
- 6.1.5 Abstract and Title
- 6.1.6 Subjects to be Excluded from the Search
- 6.1.7 Claim Interpretation, Broad Claims, PCT Articles 5 and 6
- 6.1.7.1 Claim Interpretation According to the PCT Guidelines
- 6.1.7.1.1 PCT Guideline References and Flow Chart
- 6.1.7.1.2 Overview of the Hierarchy
- 6.1.7.1.3 Special Meaning, Ordinary Meaning, Everyday Meaning
- 6.1.7.1.4 Closed and Open Definitions and Implications for Interpretation
- 6.1.7.1.5 Implications of the Hierarchy on Searching
- 6.1.7.1.6 Practices for the Interpretation of Claims
- 6.1.7.1.7 Interpretation of Citations - Inherency
- 6.1.7.2 Broad Claims
- 6.1.7.3 PCT Articles 5 and 6
- 6.1.7.4 Claims Lacking Clarity and Excessive/Multitudinous Claims
- 6.1.7.5 Procedure for Informal Communication with the Applicant
- 6.1.8 Search Strategy
- 6.1.8.1 Introduction
- 6.1.8.2 The Three Person Team (3PT)
- 6.1.8.3 Area of Search
- 6.1.8.4 Search Considerations
- 6.1.9 Basis of the Search
- 6.1.10 Non-Patent Literature
- 6.1.11 Search Procedure
- 6.1.11.1 Overview - Novelty / Inventive Step
- 6.1.11.2 Inventive Step
- 6.1.11.3 Searching Product by Process Claims
- 6.1.11.4 Dates Searched
- 6.1.11.5 Conducting the Search
- 6.1.11.6 Useful Techniques ("piggy back/forward" searching)
- 6.1.11.7 Obtaining Full Copies
- 6.1.11.8 Considering and Culling the Documents
- 6.1.11.9 Ending the Search
- 6.1.11.10 Categorising the Citations
- 6.1.11.11 Grouping the Claims
- 6.1.12 Search Report and Notification Form Completion
- 6.1.12.1 Background Search Report and Notification Form Completion
- 6.1.12.2 Applicant Details
- 6.1.12.3 General Details
- 6.1.12.4 Fields Searched
- 6.1.12.5 Documents Considered to be Relevant
- 6.1.12.5.1 Selection of Documents Considered to be Relevant
- 6.1.12.5.2 Citation Category
- 6.1.12.5.3 Citation of Prior Art Documents
- 6.1.12.5.4 Citation of URLs
- 6.1.12.5.5 Citation Examples
- 6.1.12.5.6 Citing Patent Documents Retrieved from EPOQUE
- 6.1.12.5.7 Relevant Claim Numbers
- 6.1.12.6 Family Member Identification
- 6.1.12.7 Date of Actual Completion of the Search
- 6.1.12.8 Refund Due
- 6.1.12.9 Contents of Case File at Completion
- 6.1.13 Reissued, Amended or Corrected ISRs and ISOs
- 6.1.14 Priority Document
- 6.1.15 Foreign Patent Search Aids and Documentation
- 6.1.16 Assistance with Foreign Languages
- 6.1.17 Rule 91 Obvious Mistakes in Documents
- 6.1.18 Nucleotide and/or Amino Acid Sequence Listings
- 6.1.18.1 Background Nucleotide and/or Amino Acid Sequence Listings
- 6.1.18.2 Office Practice
- 6.1.18.3 Summary
- 6.1.19 Annexes
- Annex A - Blank ISR
- Annex B - Completed ISR
- Annex C - Completed ISR
- Annex D - Declaration of Non-Establishment of ISR
- Annex E - Completed Invitation to pay additional fees
- Annex F - Completed ISR with unity observations
- Annex H - Searching Broad Claims
- Annex I - Completed notification of change of abstract
- Annex J - Completed notification of decision concerning request for rectification
- Annex K - The role of the 3 Person Team in Searching
- Annex S - Refund of Search Fees
- Annex U - ISR Quality Checklist
- Annex V - Internet Searching
- Annex W - Obtaining full text from internet
- Annex Z - USPTO kind codes
- Annex AA - Markush Claims
- Annex BB - Article 5/6 Comparisons
- 6.2 International Type Searching
- 6.2.1 Procedural Outline International Type Search Report
- 6.2.2 Introduction - International Type Searching
- 6.2.3 Classification and Search Indication
- 6.2.4 Unity of Invention
- 6.2.5 Subjects to be Excluded from the Search
- 6.2.6 Obscurities, Inconsistencies or Contradictions
- 6.2.7 Abstract and Title
- 6.2.8 Search Report
- 6.2.9 Completing Search Report and Opinion Form
- 6.2.10 Annexes
- 6.3 International Examination
- 6.3.1 Procedural Outline Written Opinion
- 6.3.2 Introduction International Examination
- 6.3.3 The Demand and IPRPII
- 6.3.4 Top-up Search
- 6.3.5 First IPE action
- 6.3.5.1 Introduction - First IPE Action
- 6.3.5.2 Supplementary International Search Report
- 6.3.5.3 PCT Third Party Observations
- 6.3.6 Response to Opinion
- 6.3.7 IPRPII and Notification
- 6.3.8 Completing ISO, IPEO and IPRPII Forms
- 6.3.8.1 Front Page and Notification Application Details
- 6.3.8.2 Box I Basis of Opinion/Report for ISOs, IPEOs and IPRPs
- 6.3.8.3 Box II Priority
- 6.3.8.4 Box III Non-establishment of Opinion
- 6.3.8.5 Box IV Unity of Invention
- 6.3.8.6 Box V Reasoned Statement Regarding Novelty, Inventive Step & Industrial Applicability
- 6.3.8.7 Box VI Certain Documents Cited
- 6.3.8.8 Box VII Certain Defects
- 6.3.8.9 Box VIII Certain Observations
- 6.3.9 General Considerations
- 6.3.9.1 Article 19 or Article 34(2)(b) Amendments
- 6.3.9.2 Formalities
- 6.3.9.3 General Notes on Form Completion
- 6.3.9.4 Rule 91 Obvious Mistakes in Documents
- 6.3.9.5 Processing withdrawals of PCTs
- 6.3.10 Annexes
- Annex A - Written Opinion-ISA
- Annex B - Written Opinion-IPEO
- Annex C - Notification of Transmittal of IPERII
- Annex D - IPRPII
- Annex E - IPRPII Clear Novel and Inventive Box V Only
- Annex F - Invitation to Restrict/Pay Additional Fees - Unity
- Annex G - Extension of Time Limit
- Annex H - IPE Quality Checklist
- Annex I - Examples of Inventive Step Objections
- Annex J - Examples of Objections under PCT Articles 5 and 6
- Annex K - Example of PCT Third Party Observations
- Annex L - Blank Written Opinion - ISA
- Annex M - Blank Written Opinion - IPEO
- Annex N - Blank IPRPII
- Annex O - ISO/ISR with Omnibus Claims
- Annex P - PCT Timeline
- Annex Z - Best Practice Examples
- 6.4 Fiji Applications
- 6.4.1 Introduction
- 6.4.2 Completion Time and Priority
- 6.4.3 Initial Processing
- 6.4.4 Search Procedure
- 6.4.5 Search Report and Advisory Opinion
- 6.4.6 Further Advisory Opinion
- 6.4.7 Final Processing
- 6.4.8 Annexes
- 6.5 Thai Applications
- 6.5.1 Introduction to Thai Applications
- 6.5.2 Completion Time and Priority
- 6.5.3 Initial Processing
- 6.5.4 Search Procedure
- 6.5.5 Search Report
- 6.5.6 Final Processing
- 6.5.7 Annex A - Thai Search Report
- 6.6 WIPO Searches
- 6.6.1 Introduction
- 6.6.2 Completion Time and Priority
- 6.6.3 Initial Processing
- 6.6.4 Search Procedure
- 6.6.5 Search Report
- 6.6.6 Final Processing
- 6.6.7 Annexes
- 6.7 Other Countries
- 6.8 PCT Articles, Regulations and Guidelines et al
- 6.9 Miscellaneous
- 7. Oppositions, Disputes and Extensions
- 7.1 Role and Powers of the Commissioner in Hearings
- 7.2 Oppositions, Disputes and other proceedings-Procedural summaries
- 7.2.1 Oppositions to grant of a standard patent-Section 59 oppositions
- 7.2.1.1 Commencing the Opposition - Filing a Notice of Opposition
- 7.2.1.2 Filing the Statement of Grounds and Particulars
- 7.2.1.3 Evidence and Evidentiary Periods
- 7.2.1.4 Finalising the Opposition
- 7.2.2 Opposition to Innovation Patents-Section 101M Oppositions
- 7.2.2.1 Commencing the Opposition - Filing the Opposition Documents
- 7.2.2.2 Evidence and Evidentiary Periods
- 7.2.2.3 Finalising the Opposition
- 7.2.3 Oppositions to an Extension of Term of a Pharmaceutical Patent (Section 75(1) Oppositions)
- 7.2.3.1 Commencing the Opposition - Filing a Notice of Opposition
- 7.2.3.2 Filing the Statement of Grounds and Particulars
- 7.2.3.3 Evidence and Evidentiary Periods
- 7.2.3.4 Finalising the Opposition
- 7.2.4 Oppositions to Request to Amend an Application or Other Filed Document (Section 104(4) Oppositions)
- 7.2.4.1 Commencing the Opposition - Filing a Notice of Opposition
- 7.2.4.2 Filing the Statement of Grounds and Particulars
- 7.2.4.3 Evidence and Evidentiary Periods
- 7.2.4.4 Finalising the Opposition
- 7.2.5 Oppositions to Extensions of Time Under Section 223 (Section 223(6) Oppositions
- 7.2.5.1 Commencing the Opposition - Filing a Notice of Opposition
- 7.2.5.2 Filing the Statement of Grounds and Particulars
- 7.2.5.3 Evidence and Evidentiary Periods
- 7.2.5.4 Finalising the Opposition
- 7.2.6 Oppositions to Grant of a Licence (Regulation 22.21(4) Oppositions)
- 7.2.6.1 Commencing the Opposition - Filing a Notice of Opposition
- 7.2.6.2 Filing the Statement of Grounds and Particulars
- 7.2.6.3 Evidence and Evidentiary Periods
- 7.2.6.4 Finalising the Opposition
- 7.2.7 Disputes Between Applicants and Co-Owners (Directions Under Section17 and Determinations Under Section 32)
- 7.2.8 Entitlement Disputes (Applications Under Sections 33-36 and 191A)
- 7.3 Directions
- 7.3.1 Directions in Opposition Proceedings
- 7.3.1.1 Direction to Stay an Opposition Pending Another Action
- 7.3.1.2 Further and Better Particulars
- 7.3.1.3 Time for Filing Evidence in a Substantive Opposition
- 7.3.1.4 Time for Filing Evidence in a Procedural Opposition
- 7.3.1.5 General Conduct of Proceedings
- 7.3.1.6 Further Directions
- 7.3.2 Directions that an Application Proceed in Different Name(s) - Section 113
- 7.4 Opposition Documents, Requirements and Amendments
- 7.4.1 Notice of Opposition
- 7.4.2 Statement of Grounds and Particulars
- 7.4.3 Amending Opposition Documents
- 7.4.4 Filing of Opposition Documents
- 7.5 Evidence
- 7.5.1 Presentation of Evidence
- 7.5.1.1 Written Evidence and Declarations
- 7.5.1.2 Oral Evidence
- 7.5.1.3 Physical Evidence - Special Considerations
- 7.5.2 Admissibility of Evidence
- 7.5.3 Evidence Filed Out of Time
- 7.6 Production of Documents, Summonsing Witnesses
- 7.6.1 Requests for Commissioner to Exercises Powers Under Section 210(1)(a) & 210(1)(c)
- 7.6.2 Basis for Issuing a Summons
- 7.6.3 Basis for Requiring Production of Documents or Articles
- 7.6.4 Reasonable Expenses
- 7.6.5 Complying with the Summons or Notice to Produce, Reasonable Excuses
- 7.6.6 Sanctions for Non-Compliance
- 7.6.7 Schedule to Requests for Summons or Notice to Produce
- 7.7 Withdrawal and Dismissal of Oppositions
- 7.7.1 Withdrawal of an Opposition
- 7.7.2 Dismissal of an Opposition
- 7.7.2.1 Requests for Dismissal
- 7.7.2.2 Dismissal on the Initiative of the Commissioner
- 7.7.2.3 Reasons for Dismissal
- 7.7.3 Withdrawal of an Opposed Application
- 7.8 Hearings and Decisions
- 7.8.1 Setting Down Hearings
- 7.8.1.1 Setting of Hearing
- 7.8.1.2 Location and Options for Appearing
- 7.8.1.3 Hours of a Hearing
- 7.8.1.4 Hearing Fee
- 7.8.1.5 Who May Appear at a Hearing?
- 7.8.1.6 Relevant Court Actions Pending
- 7.8.2 Hearings Procedure
- 7.8.2.1 Overview of Proceedings
- 7.8.2.2 Adjournment of Hearings
- 7.8.2.3 Contact with Parties Outside of Hearing
- 7.8.2.4 Hearings Involving Confidential Material
- 7.8.2.5 Consultation with Other Hearing Officers
- 7.8.2.6 Hearings and the Police
- 7.8.3 Ex Parte Hearings
- 7.8.4 Natural Justice and Bias
- 7.8.4.1 Rules
- 7.8.4.2 Waiving of Objection of Bias by Standing by until Decision Issued
- 7.8.4.3 Bias as a Result of Contact with Parties Outside of Hearing
- 7.8.4.4 Bias as a Result of Other Proceedings Involving the Same Parties
- 7.8.5 Principles of Conduct
- 7.8.5.1 Lawfulness
- 7.8.5.2 Fairness
- 7.8.5.3 Rationality
- 7.8.5.4 Openness
- 7.8.5.5 Diligence and Efficiency
- 7.8.5.6 Courtesy and Integrity
- 7.8.6 Decisions
- 7.8.6.1 Written Decisions
- 7.8.6.2 Time for Issuing a Decision
- 7.8.6.3 Publication of Decisions
- 7.8.6.4 Rectification of Errors or Omissions in Decisions
- 7.8.6.5 Revocation of Decisions
- 7.8.7 Further Hearings
- 7.8.8 Final Determinations
- 7.8.8.1 Overview of Proceedings
- 7.8.8.2 Applicant Does Not Propose Amendments
- 7.8.8.3 Opponent Withdraws the Opposition
- 7.8.9 Quality
- 7.8.10 Appointment of Hearing Officers and Assistant Hearing Officers, Hearing Officer Standards Panel, Hearing Officer Delegations
- 7.9 Costs
- 7.9.1 Principles in Awarding Costs
- 7.9.2 Scale of Costs, Variation of the Scale
- 7.9.3 Awarding Costs, Taxation
- 7.9.4 Security for Costs
- 7.9.5 Exemplary Situations in Awarding Costs
- 7.10 The Register of Patents
- 7.10.1 What is the Register
- 7.10.2 Recording Particulars in the Register
- 7.10.2.1 Recording New Particulars in the Register
- 7.10.2.2 Change of Ownership
- 7.10.2.2.1 Assignment
- 7.10.2.2.2 Change of Name
- 7.10.2.2.3 Bankruptcy
- 7.10.2.2.4 Winding Up of Companies
- 7.10.2.2.5 Death of Patentee
- 7.10.2.3 Security Interests
- 7.10.2.4 Licences
- 7.10.2.5 Court Orders
- 7.10.2.6 Equitable Interests
- 7.10.2.7 Effect of Registration or Non-Registration
- 7.10.2.8 Trusts
- 7.10.2.9 False Entries in the Register
- 7.10.3 Amendment of the Register
- 7.11 Extensions of Time and Restoration of Priority
- 7.11.1 Extensions of Time - Section 223
- 7.11.1.1 Relevant Act
- 7.11.1.2 Subsection 223(1) - Office Error
- 7.11.1.2.1 Extensions under Subsection 223(1) to Gain Acceptance
- Annex A - Section 223(1) Extension of Time for Acceptance File Note
- 7.11.1.3 Subsection 223(2) - Error or Omission and Circumstances Beyond Control
- 7.11.1.3.1 The Law
- 7.11.1.3.2 Subsection 223(2)(a) - Error or Omission
- 7.11.1.3.3 Section 223(2)(b) - Circumstances Beyond Control
- 7.11.1.3.4 Filing a Request under Subsection 223(2)
- 7.11.1.3.5 The Commissioner's Discretion
- 7.11.1.4 Subsection 223(2A) - Despite Due Care
- 7.11.1.5 Common Deficiencies in Requests under Section 223(2) or (2A)
- 7.11.1.6 Advertising an Extension - Subsection 223(4)
- 7.11.1.7 Extension of Time for an Extension of Term
- 7.11.1.8 Grace Period Extensions
- 7.11.1.9 Extension of Time to Gain Acceptance
- 7.11.1.10 Examination Report Delayed or Not Received
- 7.11.1.11 Co-pending Section 104 Application - Budapest Treaty Details
- 7.11.1.12 Payment of Continuation or Renewal Fees Pending a Section 223 Applicaiton
- 7.11.1.13 Person Concerned: Change of Ownership
- 7.11.1.14 Date of a Patent Where an Extension of Time is Granted to Claim Priority
- 7.11.2 Extensions of Time - Reg 5.9
- 7.11.2.1 Requesting an Extension of Time
- 7.11.2.2 Application of the Law
- 7.11.2.3 Justification for the Extension
- 7.11.2.4 Discretionary Matters
- 7.11.2.5 Period of an Extension
- 7.11.2.6 A Hearing in Relation to an Extension
- 7.11.2.7 Parties Involved in Negotiations
- 7.11.2.8 Review of a Decision to Grant or Refuse an Extension
- 7.11.2.9 "Out of Time" Evidence
- 7.11.3 Restoration of the Right of Priority Under the PCT
- 7.12 Extensions of Term of Standard Patents Relating to Pharmaceutical Substances
- 7.12.1 Section 70 Considerations
- 7.12.1.1 Pharmaceutical Substance per se
- 7.12.1.2 Meaning of Pharmaceutical Substance
- 7.12.1.3 Meaning of "when produced by a process that involves the use of recombinant DNA technology"
- 7.12.1.4 Meaning of "mixture or compound of substances"
- 7.12.1.5 Meaning of "in substance disclosed"
- 7.12.1.6 Meaning of "in substance fall within the scope of the claim"
- 7.12.1.7 Included in the Goods
- 7.12.1.8 First Regulatory Approval Date
- 7.12.2 Applying for an Extension of Term
- 7.12.2.1 Documentation Required
- 7.12.2.2 Time for Applying
- 7.12.2.3 Extension of Time to Apply for an Extension of Term
- 7.12.3 Processing an Application for an Extension of Term
- 7.12.3.1 Initial Processing
- 7.12.3.2 Consideration of the Application
- 7.12.3.3 Grant of Application for Extension of Term
- 7.12.3.4 Refusal of Application for Extension of Term
- 7.12.4 Calculating the Length of the Extension of Term
- 7.12.5 Patents of Addition
- 7.12.6 Divisional Applications
- 7.12.7 Oppositions to an Extension of Term
- 7.12.8 Relevant Court Proceedings Pending
- 7.12.9 Rectification of the Register
- 7.13 Orders for Inspection of non OPI Documents
- 7.13.1 Documents not-OPI by direction of the Commissioner - Regulation 4.3(2)(b)
- 7.13.2 Inspection of non-OPI Documents
- 7.14 Appeals ART, ADJR, The Courts
- 7.14.1 Appeals to the Federal Court
- 7.14.2 Administrative Review Tribunal (ART) Review
- 7.14.3 Judicial Review (ADJR)
- 7.14.4 Other Court Actions Involving the Commissioner
- 7.14.5 Section 105 Amendments
- 7.15 Computerised Decisions
- 8. Superseded Legislation and Practice
- 8.1 Summary of Relevant Legislative Changes
- 8.2 General Approach to Examination
- 8.2.1 Restriction of the Report
- 8.2.2 Not All Claims Previously Searched and/or Examined
- 8.2.3 Law and Practice Differences
- 8.3 Amendments
- 8.3.1 Allowability of Amendments to Complete Specifications
- 8.3.2 Allowability Under Section 102(1)
- 8.3.3 Allowability Under Section 102(2) - General Comments
- 8.3.4 Amendments to a Provisional Specification
- 8.3.5 Opposition to Amendments - Standard Patents
- 8.4 Novelty
- 8.4.1 Introduction
- 8.4.2 Prior Art Information
- 8.4.3 Exclusions
- 8.4.4 Doctrine of Mechanical Equivalents
- 8.4.5 Basis of the "Whole of Contents" Objection
- 8.5 Inventive Step
- 8.5.1 The Statutory Basis for Inventive Step
- 8.5.2 Prior Art Base
- 8.5.3 Assessing Inventive Step in Examination
- 8.5.4 Common General Knowledge
- 8.5.5 Determining the Problem
- 8.5.6 Identifying the Person Skilled in the Art
- 8.5.7 Could the PSA have Ascertained, Understood, Regard as Relevant and Combined the Prior Art Information
- 8.5.7.1 Ascertained
- 8.5.7.2 Understood
- 8.5.7.3 Regarded as Relevant
- 8.5.7.3.1 Document Discloses the Same, or a Similar, Problem
- 8.5.7.3.2 Document Discloses a Different Problem
- 8.5.7.3.3 Age of the Document
- 8.5.7.3.4 Would the Person Skilled in the Art Have Used the Document to Solve the Problem
- 8.5.7.4 Does the Document Constitute a Single Source of Information
- 8.5.7.5 Could the PSA be Reasonably Expected to Have Combined the Documents to Solve the Problem
- 8.6 Innovative Step
- 8.7 Section 40 Specifications
- 8.7.1 Overview
- 8.7.2 What is the Invention?
- 8.7.2.1 General Considerations
- 8.7.2.2 Approach in Lockwood v Doric
- 8.7.2.3 Consistory Clause
- 8.7.2.4 Requirement for Critical Analysis
- 8.7.2.5 "Essential Features" of the Invention
- 8.7.3 Full Description - Best Method
- 8.7.3.1 Date for Determining Full Description
- 8.7.3.2 Can the Nature of the Invention be Ascertained?
- 8.7.3.3 Compliance with Subsection 40(2) is a Question of Fact
- 8.7.3.4 Enabling Disclosures
- 8.7.3.5 Effort Required to Perform the Invention
- 8.7.3.6 Different Aspects Claimed in Different Claims
- 8.7.3.7 Inclusion of References
- 8.7.3.8 Trade Marks in Specifications
- 8.7.3.9 Colour Drawings and Photographs
- 8.7.4 Claims Define the Invention
- 8.7.5 Claims are Fairly Based
- 8.7.5.1 General Principles
- 8.7.5.2 Sub-Tests for Fair Basis
- 8.7.5.3 Relation Between the Invention Described and the Invention Claimed
- 8.7.5.4 Only Disclosure is in a Claim
- 8.7.5.5 Alternatives in a Claim
- 8.7.5.6 Claiming by Results
- 8.7.5.7 Reach-Through Claims
- 8.7.5.8 Claims to Alloys
- 8.7.6 Provisional Specifications
- 8.7.7 Complete Applications Associated with Provisional Applications
- 8.8 Patentability Issues
- 8.9 Abstracts
- 8.10 Divisional Applications
- 8.10.1 Application
- 8.10.2 Priority Entitlement
- 8.10.3 Time Limits for Filing Applications
- 8.10.4 Subject Matter
- 8.10.5 Amendment of Patent Request - Conversion of Application to a Divisional
- 8.10.6 Case Management of Divisional Applications
- 8.11 Priority Dates and Filing Dates
- 8.11.1 Priority Date of Claims
- 8.11.2 Priority Date Specific to Associated Applications (Priority Dociment is a Provisional)
- 8.11.3 Priority Date Issues Specific to Convention Applications
- 8.11.4 Priority Date Issues Relating to Amended Claims
- 8.12 Examination
- 8.13 Modified Examination
- 8.14 Petty Patents
- 8.15 National Phase Applications
- 8.15.1 Key Features of the Legislation
- 8.15.2 National Phase Preliminaries
- 8.15.3 Formality Requirements
- 8.15.4 Priority Sources
- 8.15.5 Determining Whether Amendments Made Under Articles and Rules of the PCT are Considered During Examination
- 8.15.6 Amendments During Examination
- 8.16 Convention Applications
- 8.16.1 Convention Country Listing
- 8.16.2 Convention Country Status Change
- 8.16.3 Basic Application Outside 12 Month Convention Period
- 8.16.4 Convention Priority Dates
- 8.17 Patent Deed
6.1.18.1 Background Nucleotide and/or Amino Acid Sequence Listings
Key Legislation:
Articles of the PCT:
Article 14 Certain Defects in the International Application
Article 34 Procedure Before the International Preliminary Examining Authority
Administrative Instructions under the PCT:
s208 Sequence Listings
s513 Sequence Listings
s801 Third Party Observation System
Annex C INSTRUCTIONS RELATING TO THE PRESENTATION OF NUCLEOTIDE AND AMINO ACID SEQUENCE LISTINGS IN INTERNATIONAL PATENT APPLICATIONS UNDER THE PCT
Regulations under the PCT:
Rule 5.2 Nucleotide and/or Amino Acid Sequence Disclosure
Rule 13ter Nucleotide and/or Amino Acid Sequence Listings
RIO - Processing PCT Tasks - Box I
RIO - Processing PCT Tasks - Box VII
Related Chapters:
RIO - Processing PCT Tasks - Box I
RIO - Processing PCT Tasks - Box VII
Note: As of 1 July 2022, WIPO implemented the ST.26 Standard. All applications with a filing date of 1 July onwards will need to be processed based on the procedures set out in 6.1.18.2 Office Practice and 6.1.18.3 Summary.
Introduction
Nucleotide and/or amino acid sequence listings are representations of the sequence in a standardised format set down in Annex C of the Administrative Instructions under the Patent Cooperation Treaty. This listing(s) may be supplied with an international application in addition to the sequence disclosed in the specification.
The following definitions apply:
Nucleotide sequence: an unbranched sequence of ten or more contiguous nucleotides
Amino acid sequence: an unbranched sequence of four or more contiguous amino acids
Branched sequences of either nucleotide or amino acids are specifically excluded (Annex C).
Note: A sequence listing is a separate part of the description that is presented in a specific format (Annex C). Nucleotide or amino acid sequences that appear in the figures or drawings, or in the text of the description, do not constitute a sequence listing.
Whether the listing forms part of the pamphlet
The listing may be either:
filed as part of the specification
furnished separately at a later date
In either of the following circumstances, the sequence listing forms part of the description (Rule 5.2) and hence forms part of the pamphlet:
Where the sequence listing was originally filed as part of the description
Where it was filed in response to an Article 14 objection (certain defects in the specification) by the Receiving Office
However, if the sequence listing is only filed in response to a Rule 13ter request by the International Searching Authority (ISA) then the sequence listing does not form part of the description (unless the subject of an Article 34 amendment) and will not be part of the pamphlet (Rule 13ter(f)).
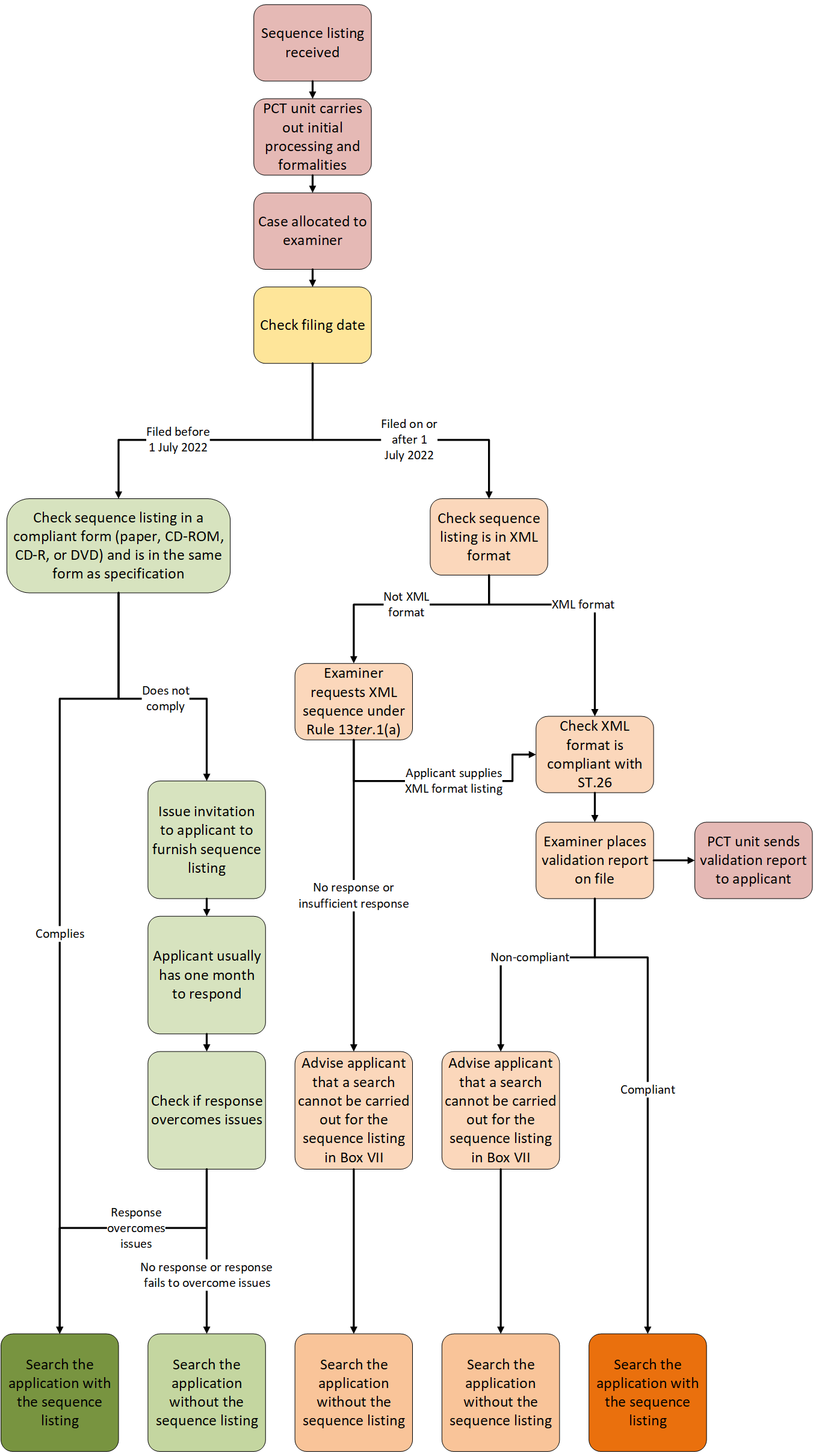
Examiner workflow: PCT applications filed on or after 1 July 2022
Note: a flow chart of the examiner workflow is provided at the end of this page.
Where the international application contains a disclosure of a nucleotide and/or amino acid sequence, it must be accompanied by sequence listing that is compliant with WIPO ST.26 Standard. The standard requires the listing to be provided in XML format.
After initial processing of the application by PCT unit, the application will be forwarded along with the attached sequence listing to the relevant examination section. The relevant examination section will then determine if the provided XML sequence listing complies with WIPO ST.26. The examiner inputs the sequence listing into the WIPO sequence validation tool which then validates whether the sequence is in the required format. The examiner then places a copy of the validation report produced by the WIPO sequence tool on file for dispatch to the applicant by the PCT unit.
Communication with applicant when requirements not met
Where the provided XML sequence listing does not comply with WIPO ST.26 standard, the applicant is to be advised via a comment in Box VII of PCT/ISA/237 (see 6.3.8.8 Box VII Certain Defects and RIO – Box VII). Examiners are to then to establish the opinion to the extent that a meaningful search could be carried out without a WIPO ST.26 compliant listing.
Where the application does not contain a separate sequence listing in XML format, examiners should request a sequence listing under Rule 13ter.1(a). The sequence listing provided must be in an electronic form and accompanied by a statement to the effect that does not include matter which goes beyond the disclosure in the international application as filed.
Considering the applicant’s response
Where a statement regarding added subject matter is not available to the competent authority, any such correction, rectification or amendment need only be taken into account by that authority for the purposes of the international search or preliminary examination to the extent that a meaningful search or preliminary examination can be carried out without such a sequence listing in electronic form.
If the applicant does not comply with an invitation, the ISA will only search the international application to the extent that a meaningful search can be carried out without the sequence listing (Rule 13ter.1(c)).
Any limitations placed upon the scope of the search, as a result of non-compliance with this request, should be discussed by the 3-person team (see 6.1.8.2 The Three Person Team (3PT)).
Note: A sequence listing provided in response to a Rule 13ter request does not form part of the international application
PCT applications filed before 1 July 2022
A full flow chart of the examiner workflow is provided at the end of this page.
Where the international application contains a disclosure of a nucleotide and/or amino acid sequence, the sequence listing may be in paper form, or electronic form on a (ISO9660) CD-ROM, CD-R or DVD. According to Section 208 of the Administrative Instructions, the standard prescribed for a sequence listing is that specified in Annex C (see Rule 5.2 and Administrative Instructions 208 & 801).
As of 1 July 2009, the filing of 'mixed mode' PCT applications (international applications where the sequence listing is filed in electronic form and the remainder of the international application is filed in paper form) is not permitted. Therefore, international applications must be filed in either paper or electronic form in order to be accepted by the Receiving Office.
When an international application containing a sequence listing is lodged with the Receiving Office, the PCT unit will check that the necessary formalities requirements have been met before the application is forwarded to the relevant examination section.
Note: An application where the sequence listing is provided in paper form may also contain a copy of the sequence listing in electronic form in order to comply with Rule 13ter (see Administrative Instruction 208 and Administrative Instruction at Annex C).
Non-compliance with formalities
If the sequence listing provided at filing is not in electronic form, or if one is provided which does not comply with Annex C, the ISA may issue an invitation to the applicant to either (under Rule 13ter.1(a)):
furnish a sequence listing in electronic form
furnish a listing which complies with Annex C
The sequence listing provided must be in electronic form encoded as a text file on CD or DVD media. The prescribed time for responding to such an invitation is usually one month. See also: RIO guidance for PCT/ISA/225, PCT Rule 13ter.1(a), and Ad. Inst. 513(a).
If the applicant does not comply with an invitation, the ISA will only search the international application to the extent that a meaningful search can be carried out without the sequence listing (see Rule 13ter.1(c)).
Any limitations placed upon the scope of the search as a result of non-compliance with this request should be discussed by the 3-person team (see 6.1.8.2 The Three Person Team (3PT)).
Amended Reasons
| Amended Reason | Date Amended |
|---|---|
Updated link to WIPO sequence validation tool |
|
Accessibility fix – alternative text for images |
|
Updated link at 'WIPO sequence validation tool' |
|
Republishing to fix image sizing of flowchart. |
|
Edited for better readability and accessibility. Edited for consistency with Style Manual. Added subheadings and On this Page menu. Fixed broken links. Added a flow chart. |
|
Added links to RIO guidance material for processing PCT tasks. |
|
Page revised to allow for restoration of content relating to IP Australia's implementation of ST.26 Standard |
|
Published for testing |
